Looking up at the night sky, you will see dozens of stars. If you live in a very dark place, you may even see hundreds. The thing that people find difficult to grasp is just how far away these stars are. Our sun is 150 million km away from earth. If you could travel at the speed of light, which is as fastest you can go, it would take 8 minutes to get there. To get to the next nearest star at the speed of light, you would be travelling for 4 years (that's without toilet stops).
Other objects are much further away. There are billions of galaxies outside of our Milky Way. Light from one of the nearest takes over 2 million years to reach earth. That is truly a galaxy far, far away. This is the Andromeda Galaxy, the farthest object you can see with the naked eye.
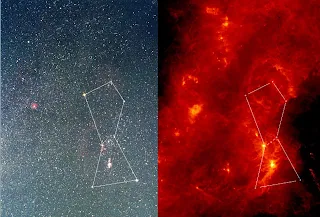
We can't get to the stars to study them, so how do we find out how they work? The answer is light. We use telescopes to collect light from the stars and watch them move and change. Some telescopes detect light that we can't see; ultraviolet, infrared, radio waves and X-rays. All of this information gives us clues about how stars work. Orion, above, looks different in visible and infrared light.
Usually, when scientists want to find out how something works, they take samples and run experiments. This is impossible with stars, as they are billions of kilometres away from Earth. How, then, can we find out what stars are made from? The answer is light again. Yes, the light from a star or galaxy contains information we can use to identify the elements in a star.
Here's how it works. If you heat up the gas of an element and put an electric current through it, it will start to glow. Each element emits light of different wavelengths. In regular English, they produce different colours. You may have seen that some street lights are orange (above) because they have sodium vapour in their bulbs. Sodium vapour produces orange light when we put an electric current through it. Other elements make different colours, neon glows red, and mercury glows blue, for example.
A spectrometer is a device which splits light up into a spectrum. You get a complete spectrum (rainbow) if the light contains all wavelengths. The spectrum of a single element is made of bright emission lines. The image above shows the emission spectra of some elements. Can you see why sodium vapour lamps glow orange? It's because the wavelengths of light emitted by sodium are in that part of the spectrum.
The image above is sunlight put through a spectroscope. It contains dark, not bright, lines. These are called Fraunhofer lines after the person who discovered them. The dark lines for each element are in the same positions as their emission lines, so we can use them to identify the elements from which the sun is made. The lines are produced by elements absorbing specific wavelengths of light, making them absorption lines.
Here's what's happening. If light passes through a gas, the elements in the gas absorb some wavelengths it. Because these wavelengths are removed, they appear as dark absorption lines in the spectrum.
Sunlight is produced deep within the core of the sun. It takes thousands of years to work its way out to the surface. Once it leaves the surface, it passes through a layer of gas called the solar atmosphere. This is what absorbs some wavelengths, producing the Fraunhofer lines. The sun's spectrum allows us to see what its atmosphere is made from.
What is causing the elements to emit or absorb light? It's all to do with the atoms the gases are made from.
Bohr was a scientist that discovered that electrons can only exist in specific positions around a nucleus. You may have heard of these being called shells. Bohr called them energy levels because an electron needs a certain amount of energy to be in one. You may be confused by the Bohr diagram of a hydrogen atom (above). You should have learned in science lessons that hydrogen only has one electron in the first shell but the diagram seems to show several shells. These are energy levels where the electron is allowed to be. The idea that hydrogen's electron is in the energy level nearest the nucleus is useful when we talk about bonding but to explain the lines in its spectrum, we need to add some other ideas.
Electrons in the energy level nearest the nucleus have the least energy. As you move out from the nucleus, each level's energy increases. Also, electrons are lazy and want to be in the lowest energy they can get. Electrons don't stay in the lowest energy level. They can jump to a higher energy level if they pick up energy. They have to receive the exact amount of energy to jump, too much or too little and stay where they are. For example, the jump from n=1 to n=2 needs less energy than from n=1 to n=3.
Photons are little packets of energy. The amount of energy they carry depends on the wavelength of the photon. You can think of photons as coming in different colours, with the colour telling you how much energy it carries; red photons have less energy than blue photons. When a photon hits an electron, it passes its energy to the electron. If the electron is hit by a photon carrying just the right amount of energy, it absorbs it and jumps to a higher energy level. The dark Fraunhofer lines in an absorption spectrum are at the wavelengths (amount of energy) that electrons need to jump from lower to higher energy levels.

What about the emission spectra, where gases release light at different wavelengths? This is still electrons moving from one energy level to another, but this time they are moving from a higher to lower energy. Electrons don't like to be in an excited state; they want to have as little energy as possible. They release a photon of light to jump from a high energy level to a lower one, producing bright lines in the spectrum. The photon's wavelength depends on the energy difference between the levels, which explains why the lines are different colours.
Hydrogen is the simplest element, with only one electron. Every other atom has multiple electrons, making their spectra more complicated. Also, the energy levels for each type of atom are slightly different because their nuclei have different numbers of protons. This is useful, though, because the spectrum of each element is unique, allowing us to identify the element producing it.
Here's the sun's spectrum again. Can you identify what it is made from?










Comments
Post a Comment